MicroRNAs (miRNAs) are small, non-coding RNA molecules about 18–25 nucleotides long that have emerged as critical regulators of gene expression in nearly all multicellular organisms. By modulating the stability and translation of messenger RNAs (mRNAs), miRNAs influence a broad range of biological processes including development, differentiation, metabolism, and cellular responses to environmental cues.
Understanding how miRNAs are produced and how they function is key to unlocking their potential in research and therapeutic applications. In this comprehensive article, we will explore the biogenesis of miRNAs, detailing the molecular machinery and cellular pathways that convert miRNA genes into functional gene silencers.
Overview of miRNA Biogenesis
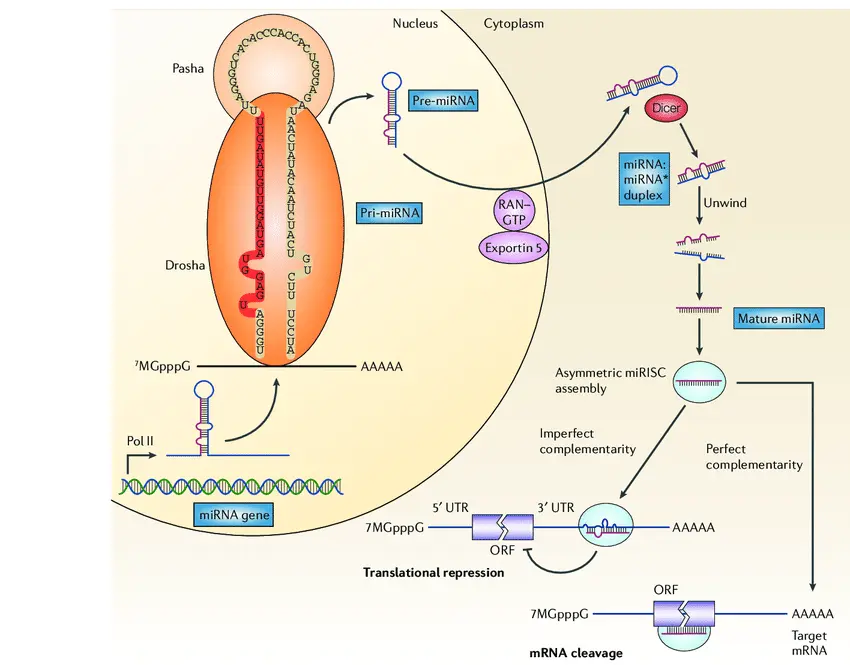
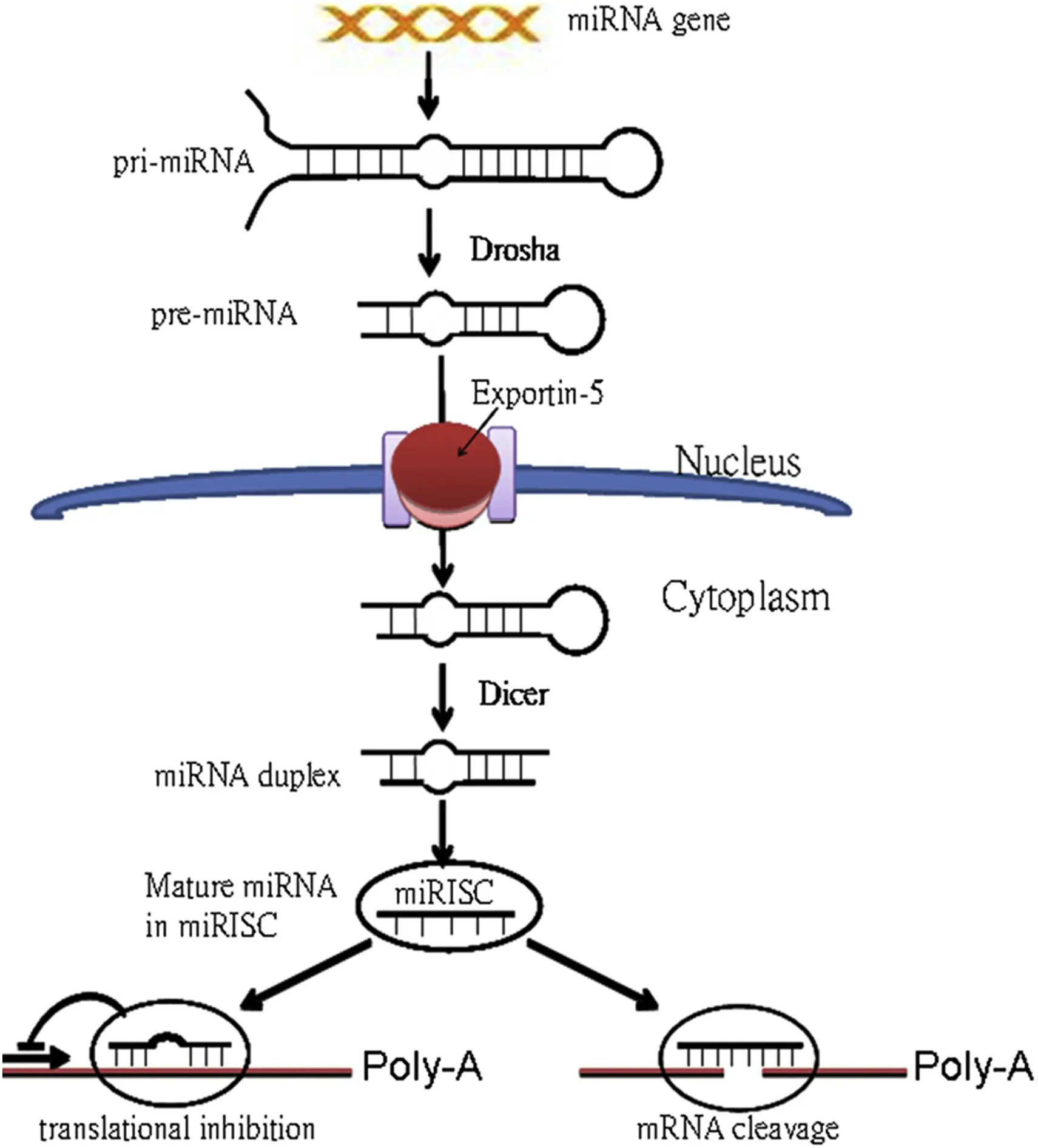
miRNA biogenesis is a multi-step process involving transcription, nuclear processing, cytoplasmic maturation, and incorporation into the gene silencing machinery. This pathway is highly conserved across species and involves an interplay of specialized enzymes and protein complexes ensuring the precise production and regulation of mature miRNAs.
Step 1: Transcription of Primary miRNAs (pri-miRNAs)
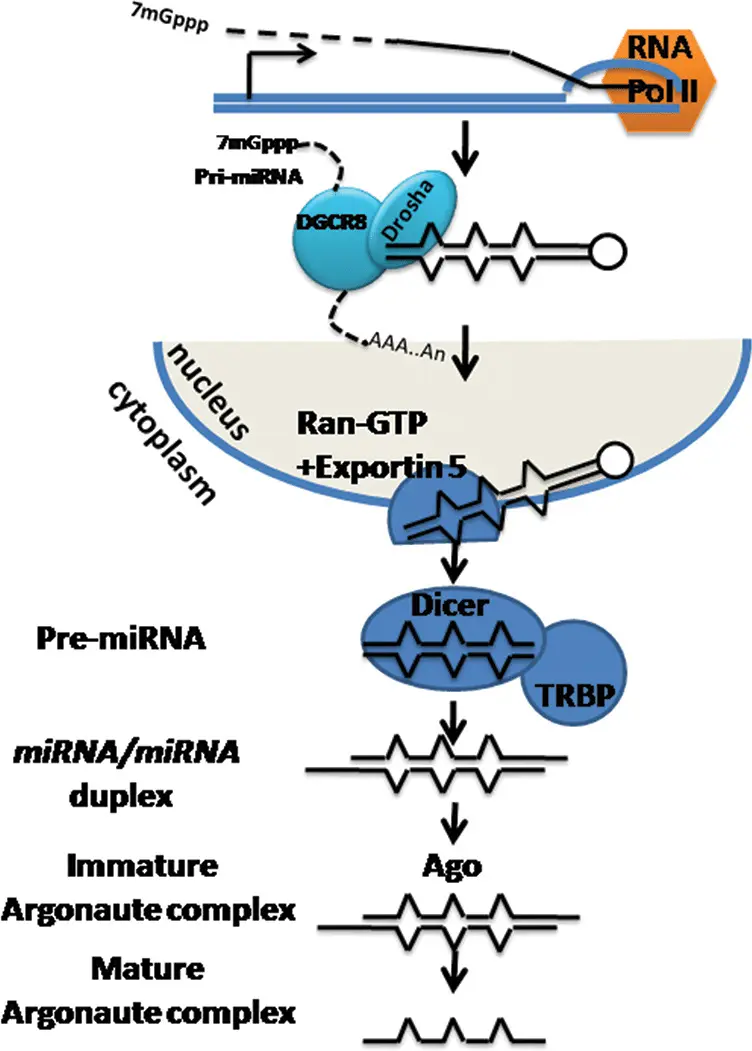
The journey of a microRNA begins in the nucleus with the transcription of miRNA genes. Most miRNAs are transcribed by RNA polymerase II, the same enzyme responsible for synthesizing messenger RNAs. This results in long primary transcripts called pri-miRNAs, which can range from hundreds to thousands of nucleotides.
These pri-miRNAs possess typical mRNA features including a 5′ cap and a polyadenylated tail, and most importantly, they contain one or more stem-loop structures that form the precursor miRNA sequences. Depending on the genomic context, miRNA genes may be:
- Intragenic: Located within introns or exons of protein-coding genes, often co-expressed with the host gene.
- Intergenic: Found in regions between genes, transcribed independently.
Some miRNAs exist as clusters, where multiple miRNA sequences are encoded within a single pri-miRNA transcript, allowing coordinated expression of functionally related miRNAs.
Step 2: Nuclear Processing by the Microprocessor Complex (Drosha and DGCR8)
The next critical step takes place in the nucleus, where the Microprocessor complex initiates pri-miRNA cleavage. This complex consists mainly of:
- Drosha: An RNase III-type endonuclease that performs site-specific cleavage.
- DGCR8 (DiGeorge Syndrome Critical Region 8): A double-stranded RNA-binding protein that recognizes and binds pri-miRNA.
Together, Drosha and DGCR8 cleave the pri-miRNA near the base of the stem-loop to release a ~70–100 nucleotide hairpin structure known as the precursor miRNA (pre-miRNA). This cropping is essential to define the boundaries of the miRNA precursor and prepare it for cytoplasmic export.
Step 3: Nuclear Export by Exportin-5
Once processed, the pre-miRNA must exit the nucleus to complete maturation. This export is mediated by Exportin-5, a nuclear transport receptor, which, in a Ran-GTP dependent manner, specifically recognizes and binds to the characteristic 2-nucleotide 3′ overhang of the pre-miRNA hairpin.
Exportin-5 ensures efficient and selective transport of pre-miRNAs to the cytoplasm while protecting them from nuclear degradation. This step links nuclear miRNA processing to cytoplasmic maturation, coordinating the two compartments.
Step 4: Cytoplasmic Processing by Dicer
In the cytoplasm, the enzyme Dicer, another RNase III family member, processes the pre-miRNA further. Dicer cleaves near the loop of the pre-miRNA hairpin, producing a short double-stranded RNA duplex approximately 18–25 nucleotides in length.
This duplex contains:
- The guide strand, which will become the functional mature miRNA.
- The passenger strand (also known as miRNA*), which is usually degraded.
The selection of the guide strand is influenced by the relative thermodynamic stability of the duplex ends; typically, the strand with a less stable 5′ end is favored.
Step 5: Assembly into the RNA-Induced Silencing Complex (RISC)
The mature miRNA strand is loaded into the RNA-Induced Silencing Complex (RISC), a multiprotein assembly responsible for gene silencing activity. The core component of RISC is an Argonaute (AGO) protein. Humans have four AGO proteins (AGO1–AGO4), with AGO2 possessing endonuclease ("slicer") activity.
Within RISC, the miRNA serves as a guide to find complementary sequences on target mRNAs. The mode of silencing depends largely on the degree of sequence complementarity:
- Perfect or near-perfect complementarity: Leads to AGO2-mediated cleavage and degradation of the target mRNA, commonly observed in plants.
- Partial complementarity: More common in animals, results in translational repression and deadenylation-mediated mRNA decay, usually through binding to the 3′ untranslated region (3′ UTR).
The Crucial Role of Argonaute Proteins
Argonaute proteins are indispensable for both miRNA stability and function. They:
- Select the correct miRNA strand for RISC incorporation
- Stabilize the miRNA within the complex
- Mediate interaction with target mRNAs
- Execute gene silencing through endonucleolytic cleavage (AGO2) or translational repression (AGO1, AGO3, AGO4)
Regulation and Variability in miRNA Biogenesis
The miRNA biogenesis pathway is highly dynamic and subject to multiple regulatory mechanisms:
- Post-translational modifications of Drosha, Dicer, and Argonaute proteins influence activity and stability.
- RNA-binding proteins can modulate processing efficiency or selectively inhibit processing of certain miRNAs.
- Cellular context such as developmental stage, tissue type, and stress conditions can shift miRNA expression patterns.
Moreover, some miRNAs undergo non-canonical biogenesis pathways, bypassing Drosha or Dicer, highlighting the complexity and adaptability of the miRNA machinery.
Conclusion: The Elegance of miRNA Biogenesis
From the initial transcription of long pri-miRNAs in the nucleus to the precise cleavage, transport, and maturation steps culminating in the assembly of the RISC effector complex, miRNA biogenesis exemplifies the intricate regulation of gene expression at the RNA level.
Each molecular player RNA polymerase II, Drosha, DGCR8, Exportin-5, Dicer, and Argonaute proteins works in concert to ensure miRNAs are produced accurately and efficiently, enabling them to exert powerful effects on cellular physiology.
For further information
Here you can find a current, comprehensive article on this topic:
Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation